Swine influenza:
variations on an old theme
Prof. Kristien Van Reeth
Laboratory of Virology, Faculty of Veterinary Medicine,
Ghent University, Belgium
Swine influenza (SI) is an
acute viral infection of the respiratory tract and an
important cause of respiratory disease in pigs. Influenza A
viruses of H1N1 and H3N2 subtypes have been circulating in
the European swine population for about 20 years. A swine
influenza virus of H1N2 subtype, however, has emerged only
recently and this has complicated the control and diagnosis
of SI.
One virus, multiple subtypes
Swine influenza viruses (SIVs) are influenza A viruses and
they belong to the family Orthomyxoviridae. These viruses
also occur in wild birds, poultry, horses and humans, but
interspecies transmission is a rare event. All influenza A
viruses have the same basic structure, but there are
considerable differences between influenza viruses of
different species and between different “subtypes” that may
co-circulate in one species. The surface of an influenza
virus particle is covered by two types of glycoproteins:
haemagglutinin (HA) and neuraminidase (NA). HA and NA are
important because they elicit an antibody response to the
influenza virus. Fifteen different HAs (H1 to H15) and 9
different NAs (N1 to N9) have been recognized by
virologists and these form the basis for classification of
influenza viruses into “subtypes”.
Pigs are notable among mammalian influenza virus hosts in
that they harbour three different subtypes: H1N1, H1N2 and
H3N2. New influenza virus subtypes can emerge in two
different ways and this is referred to as antigenic
“shift”. One possible mechanism is interspecies
transmission. The H1N1 subtype circulating in the European
swine population, for example, is originally an avian virus
that has spread from wild waterfowl into the pig population
in 1979. A second possibility is genetic “reassortment”.
This can occur when two different influenza viruses
simultaneously infect the same host. The genome of an
influenza virus is “segmented” – it comprises eight
single-stranded negative-sense RNA’s - and the 8 gene
segments of one virus can then mix and match with that of
the other virus to form a new combination virus. Both the
H3N2 and the H1N2 SIVs circulating in Europe are
reassortants. The H3N2 virus is human in origin and it’s HA
and NA resemble those of the human virus that caused the
“Hong Kong flu” pandemic in 1968. This virus has later
reassorted with the “avian-like” swine H1N1 virus, from
which it derived the internal proteins. The H1N2 virus was
first isolated in Great Britain in 1994 and has a very
complex history of origin. The virus appears to be a
combination of the HA of a human H1N1 virus from the early
1980’s and the NA of the swine H3N2 virus. The internal
proteins, on the other hand, are “avian-like” as in the
swine H1N1 and H3N2 viruses. The European H1N2 virus,
therefore, is remarkably distinct from the H1N2 virus that
has been previously reported in Japan and from H1N2 in the
US.
Smaller antigenic changes in the HA or NA of an already
existing influenza virus subtype are called antigenic
“drift”. Generally spoken, the immunity that develops after
infection with a given influenza virus subtype protects
only partially against a “’drift variant” and not at all
against a new subtype. Antigenic drift is less common with
swine than with human influenza viruses. Still, genetic
reassortment of influenza viruses appears to occur more
frequently in pigs than in any other influenza virus host.
This can be explained by the fact that pigs are susceptible
to infection with a variety of influenza A virus strains,
both of avian and human origin. In addition, pigs have
regular close contacts with humans and birds.
Three SIV subtypes circulate concurrently in Europe
SIV spreads readily by the air and by
contact with respiratory secretions from infected pigs. It
is therefore impossible to prevent SIV infections by
sanitary measures alone, and the virus is enzootic in most
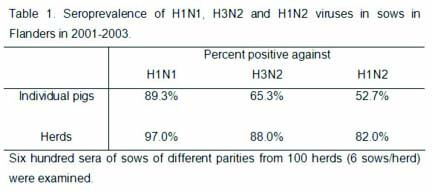
densely swine populated regions of Europe. Table 1 shows
the seroprevalence of H1N1, H3N2 and H1N2 SIVs in
unvaccinated sows in Flanders, Belgium, between 2001 and
2003. In this region, only one out of 100 farms sampled was
completely negative for any SIV subtype. Most individual
sows had antibodies to a combination of two (48%) or to all
three (31%) subtypes, indicating infection with multiple
subtypes during their lifetime. A similar situation has
been reported in Germany, Italy and Spain, where the three
subtypes are also widespread.
Most pigs are thus born from immune sows and will have
maternally-derived antibodies during their first 7 to 8
weeks of life. If pigs become exposed to the influenza
virus in the presence of maternal antibodies, they may be
(partially) protected against the infection and they are
less likely to develop disease. Most SIV infections,
therefore, occur after 10 weeks of age. By the end of the
fattening period, however, pigs rarely test negative for
SIV antibodies. In recent serologic examinations at the
slaughterhouse in Flanders, only 9% of the 168 pigs
examined were SIV negative and 44% had antibodies to two
different SIV subtypes.
The disease
SIV is one of the rare primary respiratory
pathogens of swine. This means that the virus can induce
disease and lung lesions on its own. Typical swine “flu”
outbreaks are characterized by a rapid onset of high fever,
dullness, loss of appetite, labored abdominal breathing and
coughing. Weight loss can be considerable, but mortality is
low and recovery occurs within 7-10 days. In studies of
acute respiratory disease outbreaks on pig farms in the
Netherlands in 1996-1998, SIV was diagnosed in nearly 50%
of the cases. All three virus subtypes have been associated
with disease and there are no indications for differences
in virulence between subtypes or strains. However, the
infection is much more frequent than the disease.
Subclinical infections are very common and many pigs become
infected with the virus without ever showing clinical
signs. Another possibility is that the infection with SIV
does not induce the typical flu symptoms, but that it
interacts with other respiratory pathogens to induce
multifactorial disease. Mycoplasma hyopneumoniae, porcine
reproductive and respiratory syndrome virus (PRRSV), P.
multocida, H. parasuis, S. suis and B. bronchiseptica have
been found in association with SIV and SIV is considered a
contributor to the so-called porcine respiratory disease
complex (PRDC). Also, secondary infections with bacteria
may cause more severe and prolonged disease and even
mortality in typical clinical cases of SI.
Understanding disease mechanisms
From the pathogenetic viewpoint, SIV is the typical example
of an acute respiratory tract infection. The virus
replicates in epithelial cells of the entire respiratory
tract, from the nose to the deeper lungs, but almost never
enters other tissues. Immunofluorescence and
immunohistochemical studies have shown that bronchiolar and
alveolar epithelial cells are the major target cells (Fig.
1). Epithelial cell necrosis and an influx of neutrophils
into the lung accompany the typical respiratory disease.
These inflammatory cells cause obstruction of the airways
and substantial lung damage by release of their enzymes.
Both the infection and disease are very transient. Virus
excretion in nasal swabs and virus replication in the lungs
last for 6-7 days at most.
Why does not every infection with SIV result in the typical
clinical symptoms? Based on our experiments, we believe
that disease severity relates to the amount of virus
produced in the lungs and the resulting release of
inflammatory mediators by the host. Under experimental
conditions, the infection can be easily reproduced, but the
typical disease and lung pathology only result when pigs
are inoculated with high virus doses directly into the
trachea. In such intratracheal infection studies, we found
massive virus titers in the lungs and high levels of
several cytokines, or “signal molecules”, in lung lavage
fluids. The cytokines included interferon-alpha
(IFN-),
tumor necrosis factor-alpha (TNF-),
interleukin-1 (IL-1) and IL-6. These cytokines are known to
cause lung inflammation, functional lung disturbances,
fever, malaise and loss of appetite, and they can strongly
enhance each other’s effects. These symptoms were seen
within 24 hours after intratracheal inoculation of pigs
with SIV and they were associated with the peak of virus
replication and peak cytokine levels. In contrast,
inoculation by the less invasive intranasal or aerosol
inoculation routes resulted in lower virus titers in the
lungs. These infections remained clinically mild or
subclinical and failed to induce the massive production of
cytokines in the lungs (Fig. 2). It is noteworthy that
intratracheal infection studies with other respiratory
viruses, such as the porcine respiratory corona virus
(PRCV) or PRRS virus, also failed to induce high levels of
multiple cytokines as well as obvious respiratory disease.
Together, these data support that a massive SIV replication
in the lungs is required to induce high cytokine levels and
the associated disease. Any factors (partial active or
passive immunity, sanitary measures…) likely to reduce the
extent of virus replication are thus likely to prevent
disease.
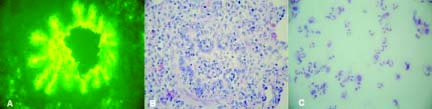
Fig. 1.
Lungs of a pig 24 hours after experimental infection with
SIV. (A) Immunofluorescence staining of bronchiolar
epithelium, (B) Neutrophil infiltration in the bronchiolar
lumen, (C) Neutrophils are the predominant cells in lung
lavage fluids.
Effectiveness of the current SI vaccines
Commercial, inactivated SIV vaccines for intramuscular
administration have been in use in Europe since the early
1980s. They are based on whole inactivated influenza virus,
or its immunogenic proteins, HA and NA, and an oil
adjuvant. Most vaccines used in Belgium and in the
Netherlands contain the human New Jersey/76 (H1N1) and Port
Chalmers/73 (H3N2) strains, but none of the vaccines
contains an H1N2 component. The first vaccination, usually
around the age of 10 weeks, should consist of two
injections 3 to 4 weeks apart. In sows, bi-annual
revaccinations are recommended. These inactivated vaccines
rely largely on circulating antibodies to the viral HA for
protection. There appears to be some diffusion of these
antibodies from the circulation into the lungs, where they
can neutralize the virus in case of an infection.
Consequently, there is a tight correlation between HI
antibody titers induced by the vaccine and protection.
Of all vaccines against respiratory viruses of pigs, SI
vaccines are among the most effective. Under experimental
conditions, a double vaccination of SIV seronegative pigs
can provide complete protection against a severe
intratracheal challenge with a high dose of H1N1 or H3N2
SIV. In our experiments, pigs that were not completely
protected against the infection show a highly significant
reduction of the challenge virus titer in their lungs when
compared to unvaccinated control pigs. Cytokine titers in
the lungs of these vaccinated pigs were also 10 to 100
times lower than in the control pigs, and all vaccinated
pigs were completely protected against disease. This led us
to conclude that even a minimal reduction of the influenza
virus titer in the lungs of vaccinated pigs strongly
reduces cytokine levels and that this reduction is
sufficient to prevent the typical disease.
Another important point is that the commercial vaccines,
which contain older H1N1 and H3N2 strains, do still
adequately protect against the H1N1 and H3N2 strains that
are currently circulating in swine. There has been much
debate as to whether antigenic drift of H1N1 and H3N2 SIVs
would require replacement of the vaccine strains by more
recent ones, as occurs with human and equine flu vaccines.
Our and other experimental studies have convincingly shown
that such a replacement is not needed. One weakness of the
current vaccines, however, is that they did not protect
against infection or disease by the H1N2 virus in our
experiments. This can be explained by the dramatic
antigenic difference between the H1 of H1N1 and H1N2
viruses. As such, the antibodies induced by the H1N1
vaccine strain cannot neutralize the H1N2 virus and
therefore not prevent the infection. Thus, some
consideration should be given to the inclusion of an H1N2
strain in SIV vaccines for use in pigs in Europe.
In the field, the efficacy of SI vaccination will depend on
numerous factors. Maternal antibodies, for example, may
interfere with effective vaccination of piglets. This is
one reason why SI vaccines are mainly used in sows, but in
most European countries less than one quarter of the sow
population is vaccinated. Though there is little published
efficacy data on SI vaccination, serologic investigations
have shown significantly higher H1N1 and H3N2 HI antibody
titers, up to 640-2560, in vaccinated than in unvaccinated
sows. Sow vaccination clearly results in higher maternal
antibody levels in the young piglets, which may persist
until the age of 12-14 weeks. It will control disease in
suckling pigs and seems to protect pigs throughout the
nursery phase.
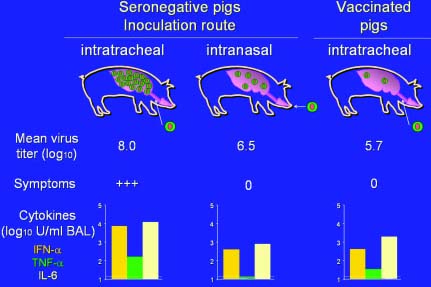
Fig. 2. SIV titers in the pig lung, clinical
outcome and cytokine levels (log10 values) in lung lavage
fluids in 3 different experimental situations: (1)
intratracheal inoculation of SIV seronegative pigs, (2)
intranasal inoculation of SIV seronegative pigs, (3)
intratracheal inoculation of previously vaccinated pigs.
The results suggest that any measures that reduce the
infection pressure and/or the viral load in the lungs will
reduce cytokine production and the resulting symptoms.
Some cross-protection between subtypes after
infection with SIV
Apart from protection following vaccination,
we have also studied the protective immune response after a
true infection with SIV. We have focused on the question as
to whether pigs with infection-derived immunity to H1N1 or
H3N2 are still fully susceptible to the antigenically
different H1N2 virus. To examine this, several groups of
pigs were inoculated intranasally with two (H1N1-H1N2,
H3N2-H1N2) or three subtypes (H1N1-H3N2-H1N2) one month
apart. H1N2 challenge virus titers in nasal swabs were
determined to evaluate protection against challenge. Serum
HI antibody titers to each of the three subtypes were also
determined at several time points. These data were used to
gain insights in the interpretation of serological profiles
from the field.
Prior infection with H1N1 or H3N2 did not protect against
infection with the H1N2 virus, while H1N2 infection-immune
pigs were fully protected. Still, H1N2 virus excretion was
up to two days shorter in H1N1 or H3N2 immune pigs than in
fully naïve pigs. This points towards a very limited and
partial cross-protection between two different SIV
subtypes. A surprisingly solid cross-protection to the H1N2
virus was found in pigs with infection-immunity to both
H1N1 and H3N2. The H1N2 virus was not or barely detectable
in nasal swabs as well as in lung tissue of these pigs.
Following intratracheal H1N2 challenge, the pigs did not
show any clinical signs. Despite the protection against the
H1N2 subtype, these pigs only had antibodies to H1N1 and
H3N2 at the time of the H1N2 challenge. There was thus no
serological cross-reaction between the subtypes in the HI
test.
Thus, though cross-protection between subtypes does not
occur after vaccination, it may be induced by exposure to
infectious SIV. This suggests that viral proteins other
than the HA, which is the major immunogenic protein in the
vaccines, may have some role. A mucosal or cell-mediated
immune response, which is only stimulated by infection, is
probably involved.
The exact significance of these experimental findings for
the field situation is not yet clear. It is possible that
this “heterosubtypic” protection contributes to the mild
clinical course of many SIV infections in the field.
Diagnosis
The diagnosis of SIV is relatively
straightforward, but the timing of sample selection is a
critical issue. Because SIV replicates in the respiratory
tract for only 5 to 7 days after infection, samples should
be collected from febrile, acutely affected pigs. Both lung
tissue or nasal swabs can be used to isolate the virus. In
the diagnostic laboratory, embryonated chicken eggs or cell
cultures are used for virus isolation. Virus isolation
remains among the most sensitive detection methods, but it
takes at least several days and up to 1-2 weeks to
determine the SIV subtype. The fluorescent antibody test on
frozen sections of lung is a more rapid (2-3 hours)
alternative. Because only small portions (4 mm sections) of
lung are examined in this test, it is less sensitive than
virus isolation. A commercial membrane immunoassay
(Directigen TM, Becton
Dickinson) to demonstrate influenza virus in nasal swabs is
also available. This test was designed to detect influenza
viruses in throat and nasal swabs of humans, but is works
equally well for any influenza A virus from other species,
including swine, poultry and horses. Speed is the greatest
advantage of this test because it takes only 20 minutes,
but it is not subtype-specific.
The haemagglutination inhibition (HI) test is the most
widely used serologic test for SIV. HI antibody titres in
serum peak by 2-3 weeks after an infection and they begin
to decline by 3-6 months. Paired sera, collected at the
time of the presumed SIV outbreak (acute serum) and
approximately 3 weeks later (convalescent serum), are
needed for the serologic diagnosis of SIV. If there has
been a recent infection with a given SIV subtype, the
convalescent sera will show rising antibody titers to that
subtype. The HI test is highly subtype-specific and
separate tests must be performed with H1N1, H3N2 and H1N2
strains as antigens. Furthermore, the test is most
sensitive if these strains resemble the current field
strains.
Our pig infection experiments with multiple SIV subtypes
have confirmed the lack of serologic cross-reaction between
subtypes. HI antibodies to a given SIV subtype generally
point towards a previous infection with that subtype. On
the other hand, the interpretation of HI antibody profiles
may become much more complex if pigs have been subsequently
exposed to different SIV subtypes. To illustrate, pigs that
become infected with H1N1 followed by H1N2 or vice versa,
may show a serologic booster to the first infecting subtype
after the second infection. The other way round,
seroconversion to a given SIV subtype can be less
pronounced if pigs are partially protected against that
subtype.
SIV: a zoonotic risk?
As mentioned higher, influenza viruses are
species-specific and this is also true for swine influenza
viruses. There are only a few documented cases of
transmission of SIVs to humans, often children. In these
rare cases, there was no further transmission of SIV
between humans. For a still unknown reason, SIVs do not
manage to spread efficiently in the human population. The
single exception here was the so-called “New Jersey”
incident in the US in 1976, during which some 500 humans
became infected with a swine H1N1 influenza virus. Still,
most of these infections were subclinical and there was no
real swine flu pandemic. Recent serologic investigations
have shown higher levels of seropositivity to H1N1 SIV in
people who had close contact with pigs than in urban
residents. However, these data must be interpreted with
caution, because it is difficult to distinguish between
antibodies to human and swine influenza viruses by the
classical serologic methods.
One difference between swine and other mammalian influenza
virus hosts is that swine are clearly more susceptible to
infection with avian influenza viruses. In the past, it was
assumed that spread of avian influenza viruses into the
human population can only occur after passage or
reassortment of these viruses in the pig, but this
hypothesis had to be rejected recently. Indeed, some of the
so-called “highly pathogenic” avian influenza viruses may
occasionally transmit directly to humans. Recent examples
are the avian H5N1 virus in Hong Kong in 1997 and in
Vietnam and Thailandin 2004-05, as well as the H7N7 virus
in The Netherlands in 2003. Interestingly, the H7N7 virus
was also found in a limited number of swine at the time of
the avian flu outbreak, and the H5N1 virus had been
occasionally detected in pigs in China in 2001 and 2003.
However, transmission of such viruses between pigs appears
to be minimal and both viruses disappeared from the swine
population. Most important, both swine and humans appear to
have been infected directly by poultry, by close contact
with infected chickens. It remains uncertain, therefore,
whether swine can play a role in the transmission of avian
influenza viruses to humans and this question definitely
merits more research.
Conclusions
The establishment of the H1N2 virus in the
European swine population has added an extra level of
complexity to the control and serologic diagnosis of SI in
Europe. To afford maximum protection, SI vaccines may need
to contain all three SIV subtypes. On the other hand, some
cross-subtype protection seems to occur following natural
SIV infections. The presence of a third SIV subtype is thus
not necessarily detrimental. Finally, continuous
surveillance of the swine population for influenza viruses
remains essential, because the epidemiological situation
can change rapidly.
Selected references
BROWN I.H., HARRIS P.A., McCAULEY J.M.,
ALEXANDER D.J.: Multiple genetic reassortment of avian and
human influenza A viruses in European pigs resulting in the
emergence of an H1N2 virus of novel genotype. J. Gen.
Virol., 1998, 79, 2947-2955.
BROWN I.H.: The epidemiology and evolution of influenza
viruses in pigs. Vet. Microbiol., 2000,
74, 29-46.
CAMPITELLI L., DONATELLI I., FONI E., et al. :
Continued evolution of H1N1 and H3N2 influenza viruses in
pigs in Italy. Virology, 1997, 232,
310-318.
CLAAS E.C.J.: Pandemic influenza is a zoonosis, as it
requires introduction of avian-like gene segments in the
human population. Vet. Microbiol., 2000,
74, 133-139.
DE JONG J.C., VAN NIEUWSTADT A.P., KIMMAN T.G., et al.:
Antigenic drift in swine influenza H3 haemagglutinins with
implications for vaccination policy. Vaccine, 1999,
17, 1321-1328.
DE JONG J.C., HEINEN P.P., LOEFFEN W.L., et al.: Antigenic
and molecular heterogeneity in recent swine influenza A
(H1N1) virus isolates with possible implications for
vaccination policy. Vaccine, 2001, 19,
4452-4464.
HAESEBROUCK F., PENSAERT M.B.: Effect of intratracheal
challenge of fattening pigs previously immunised with an
inactivated influenza H1N1 vaccine. Vet. Microbiol., 1986,
11, 239-249.
HEINEN P.P., VAN NIEUWSTADT A.P., POL J.M., et al.:
Systemic and mucosal isotype-specific antibody responses in
pigs to experimental influenza virus infection. Viral.
Immunol., 2000, 13, 237-247.
HEINEN P.P., DE BOER-LUIJTZE E.A., BIANCHI A.T.J.:
Respiratory and systemic humoral and cellular immune
responses of pigs to a heterosubtypic influenza A virus
infection. J. Gen. Virol., 2001, 82,
2697-2707.
HEINEN P.P., VAN NIEUWSTADT A.P., DE BOER-LUIJTZE E.A.,
BIANCHI A.T.J.: Analysis of the quality of protection
induced by a porcine influenza A vaccine to challenge with
an H3N2 virus. Vet. Immunol. Immunopathol., 2001,
82, 39-56.
LARSEN D.L., KARASIN A., ZUCKERMANN F., OLSEN C.W.:
Systemic and mucosal immune responses to H1N1 influenza
virus infection in pigs. Vet. Microbiol., 2000,
74, 117-131.
LOEFFEN W.L.A., KAMP E.M., STOCKHOFE-ZURWIEDEN N., et al.:
Survey of infectious disease agents involved in acute
respiratory disease in finishing pigs. Vet. Rec., 1999,
145, 175-180.
MAROZIN S., GREGORY V., CAMERON K., et al.: Antigenic and
genetic diversity among swine influenza A H1N1 and H1N2
viruses in Europe. J. Gen. Virol., 2002,
83, 735-745.
MURTAUGH M.P., BAARSCH M.J., ZHOU Y., SCAMURRA R., LIN G.:
Inflammatory cytokines in animal health and disease. Vet.
Immunol. Immunopathol., 1996, 54, 45-55.
OLSEN C.W., BRAMMER L., EASTERDAY B.C., et al.: Serologic
evidence of H1 swine influenza virus infection in swine
farm residents and employees. Emerg. Infect. Dis., 2002,
8, 814-819.
SCHRADER C., SUSS J.: Genetic characterization of a porcine
H1N2 influenza virus strain isolated in Germany.
Intervirology, 2003, 46, 66-70.
SWENSON S.L., VINCENT L.L., LUTE B.M., et al.: A comparison
of diagnostic assays for the detection of type A swine
influenza virus from nasal swabs and lungs. J. Vet. Diagn.
Invest., 2001, 13, 36-42.
VAN REETH K., NAUWYNCK H., PENSAERT M.: Bronchoalveolar
interferon,
tumor necrosis factor,
interleukin-1, and inflammation during acute influenza in
pigs: a possible model for humans? J. Infect. Dis., 1998,
177, 1076-1079.
VAN REETH K., BROWN I.H., PENSAERT M.: Isolations of H1N2
influenza A virus from pigs in Belgium. Vet. Rec., 2000,
146, 588-589.
VAN REETH K., LABARQUE G., DE CLERCQ S., PENSAERT M.:
Efficacy of vaccination of pigs with different H1N1 swine
influenza viruses using a recent challenge strain and
different parameters of protection. Vaccine, 2001,
19, 4479-4486.
VAN REETH K., VAN GUCHT S., PENSAERT M.: Correlations
between lung proinflammatory cytokine levels, virus
replication and disease after swine influenza virus
challenge of vaccination-immune pigs. Viral Immunol., 2002,
15, 583-594.
VAN REETH K., GREGORY V., HAY A., PENSAERT M.: Protection
against a European H1N2 swine influenza virus in pigs
previously infected with H1N1 and/or H3N2 subtypes.
Vaccine, 2003, 21, 1375-1381.
VAN REETH K., VAN GUCHT S., PENSAERT M.: Investigations of
the efficacy of European H1N1- and H3N2-based swine
influenza vaccines against the novel H1N2 subtype. Vet.
Rec., 2003, 153, 9-13.